EVOLUTION OF HYDROGEN FROM TUNGSTEN TRIOXIDE ELECTRODES VIA THE PHOTOLYSIS OF WATER
By: Sam Cremins
Week 1
My project will be to make tungsten trioxide electrodes which will then be used to harness the energy from sunlight to split water through the process of photolysis, where energy from photons is used to break chemical bonds. The end result will be the production of hydrogen from water using sunlight. Which in itself is a sustainable zero carbon process for producing a renewable energy source.
Week 2
Experiment 1 - First Experiment was started today and involved the preparation of one of the components needed for the experiment by dissolving solid Dowex, an ion exchange resin, in acid and leaving it over-night. This resin will then be used in later experiments to prepare some of the other components that will be needed for the experiments main section. The solution was left stirring with a magnetic stirrer for around 24 hours until everything was stable and thoroughly mixed.


Week 3 & 4
In the second week of lab sessions a complete test run of the process of making the electrodes for the project was done from start to finish. This was from making the ion exchange collum to produce the correct acid to use for the synthesis of the tungsten trioxide to coat the electrode, to the cutting and clean of the electrodes themselves using process such as ultrasonic probing, ion exchange and rotatory evaporation.

Week 5
During this week no lab progress was made with both me and my project leader being busy with other matters. Instead this week was used for mainly project research into some papers from the American Chemical Journal which followed the same research lines as my project. This gave me a good insight into how the experiment was conducted and assured me that I was on the right lines for my process.
Week 6
Preparing for the production of the F68 electrode, which will be used to conduct the testing for this project was the goal of my week. Having learnt from a few practice runs of the experiment as well as perfecting some new experimental techniques I was beginning to feel confident about producing a good F68 electrode to gather some data for the report.
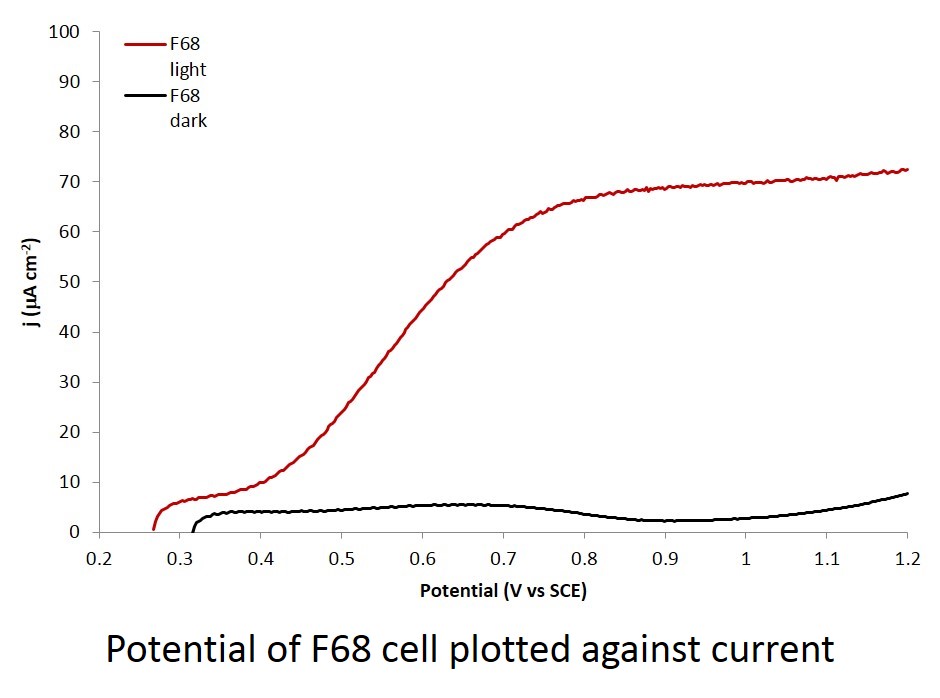
Week 7
After the last 2 weeks being slow, repetitive, following of the same process, the making of the electrodes, testing of them can finally begin. Now all the polymers have been added to the tungsten trioxide solutions and each polymer now has its own electrode. The PEG 300 electrode took the longest period of time to produce as it was the first one in the process therefore lots of experimental techniques needed to be practiced and improved for good electrode to be produced.
Week 8
In the final week testing of the electrodes has commenced with the Raman lasers being employed the check whether or not the crystal structure of the surface of the electrodes was mono-clinic, a requirement for them to be useful for this experiment. The UV lamp and special cell were then used to test for the presence of a photocurrent. If a current was detected to be flowing between the electrodes when exposed to the UV rays, then the tungsten trioxide was indeed harness the energy from the rays.